
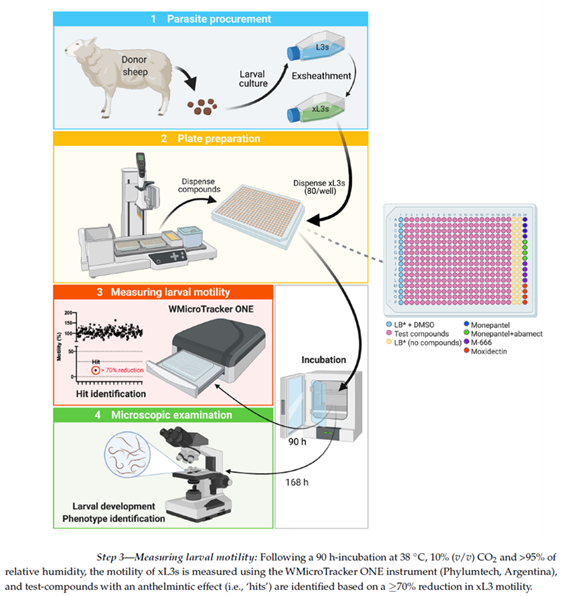
Parasitic worms cause very significant diseases in animals and humans worldwide, and their control is critical to enhance health, well-being and productivity. Due to widespread drug resistance in many parasitic worms of animals globally, there is a major, continuing demand for the discovery and development of anthelmintic drugs for use to control these worms. Here, we established a practical, cost-effective and semi-automated high throughput screening (HTS) assay, which relies on the measurement of motility of larvae of the barber’s pole worm (Haemonchus contortus) using infrared light-interference.
High Throughput Screening of Compounds for xL3 Motility Reduction at 90 h (Fig.6-step 3). After 90 h of incubation of xL3s with compounds (20 µM), larval motility was measured for 15 min in individual wells of each plate by infrared light beam-interference using a WMicroTracker ONE instrument (Phylumtech, Sunchales, Argentina). Raw data captured were normalised against measurements obtained for the positive (M-666) and negative (LB* + 0.4% DMSO) -controls to remove plate-to-plate variation by calculating the percentage of motility using the program GraphPad Prism v.9.1.0 (GraphPad Software, San Diego, CA, USA). A compound was considered active (‘hit’) if it reduced larval motility by ≥70%.
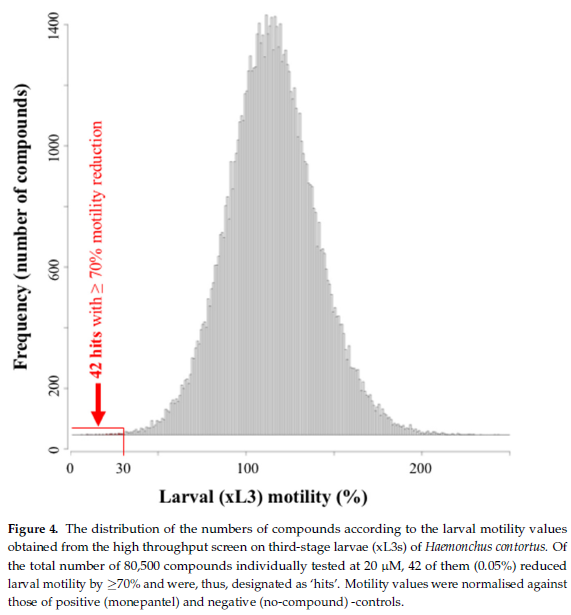
Using the established assay, we screened the 80,500 compounds from the Jump-stARter library at a concentration of 20 M. At 90 h, we identified 42 compounds that reduced xL3 motility by 70%, equating to an overall ‘hit rate’ of 0.05% (figure 4). We selected three hit candidates (i.e., UoM-8811, UoM-7024 and UoM-8035) with xL3 motility reductions of between 72% and 91% at 90 h in the primary screen for further evaluation in the dose-response assay. At 168 h, UoM-8811, UoM-7024 and UoM-8035 markedly inhibited larval development in this assay, achieving IC50 values of ~4 µM, 25 µM and 41 µM, respectively, and induced abnormal phenotypes (Cur and Evi). As there is no previous report of any of these three compounds having been tested against a parasitic nematode, current work is focused on their optimization by medicinal chemistry, followed by subsequent structure–activity relationship (SAR), bioavailability and toxicity evaluations.
Pharmaceuticals 2021, 14, 616. https://doi.org/10.3390/ph14070616
Aya C. Taki, Joseph J. Byrne, Tao Wang, Brad E. Sleebs, Nghi Nguyen, Ross S. Hall, Pasi K. Korhonen, Bill C.H. Chang, Paul Jackson, Abdul Jabbar, and Robin B. Gasser.
