
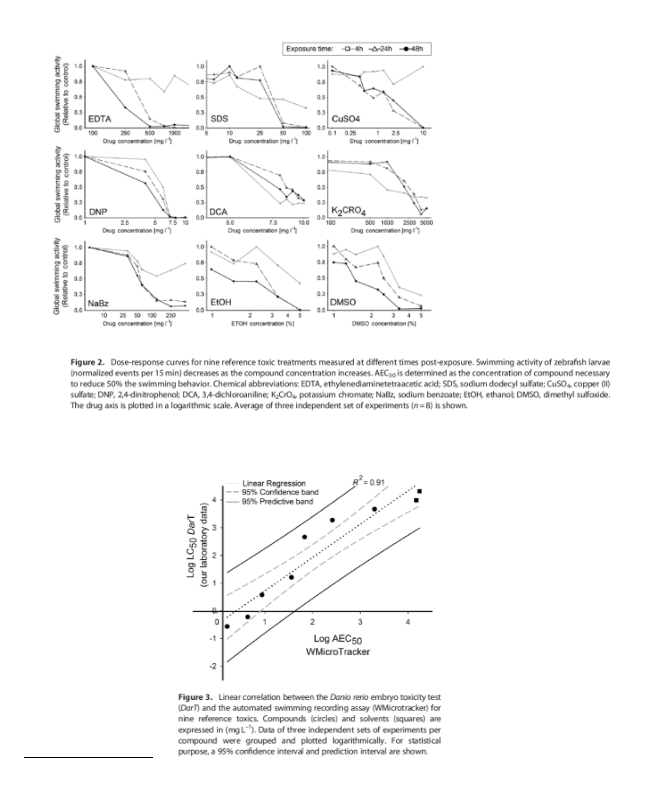
Zebrafish (Danio rerio) is increasingly employed for evaluating toxicity and drug discovery assays. Commonly experimental approaches for biotoxicity assessment are based on visual inspection or video recording. However, these techniques are limited for large-scale assays, as they demand either a time-consuming detailed inspection of the animals or intensive computing resources in order to analyze a considerable amount of screenshots. Recently, we have developed a simple methodology for tracking the locomotor activity of small animals cultured in microtiter plates. In this work, we implemented this automatic methodology, based on infrared (IR) microbeam scattering, for measuring behavioral activity in zebrafish larvae. We determined the appropriate culture conditions, number of animals and stage of development to get robust results. Furthermore, we validated this methodology as a rapid test for evaluating toxicity. By measuring the effects of reference compounds on larvae activity, we were able to estimate the concentration that could cause a 50% decrease in activity events values (AEC50), showing a Q1 strong linear correlation (R2 = 0.91) with the LC50 values obtained with the standard DarT test. The toxicity order of the measured compounds was CuSO4>2,4-dinitrophenol>3,4-dichloroaniline>SDS>sodium benzoate EDTA>K2CrO4; regarding solvents, EtOH_DMSO.
Materials and Methods:
In this study, we demonstrate that global swimming behavior could be a simple readout for toxicity, easy to scale-up in automated experiments. This approach is potentially applicable for fast ecotoxicity assays and whole-organism high-throughput compound screening, reducing the time and money required to evaluate unknown samples and to identify leading pharmaceutical compounds.
Three 48-hpf non-hatched zebrafish embryos were placed in each well of a 96-well plate containing 225 ml of E3 medium and incubated for additional 48 h at 28 C. Twenty-five mircoliters of a 10-fold concentrated compound solution or E3 medium (control) were added to complete a 250-ml final volume. For each assay, eight technical replicates were used for each dilution. Activity events were recorded during 15 min at 4, 24 and 48 h after the addition of the tested compound at room temperature and immediately after, the plates were checked by manual inspection at a stereoscopic microscope, in order to validate the activity count. Experiments were initiated between 3 and 5 h after ambient illumination went on (ZT3 to ZT5).
Results:
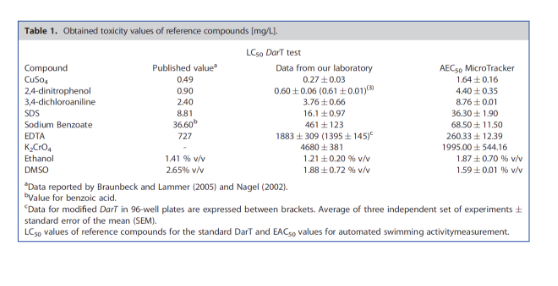
The toxicity order of the measured compounds was CuSO4 > 2,4-dinitrophenol > 3,4-dichloroaniline > SDS > sodium benzoate EDTA > K2CrO4; regarding solvents, EtOH DMSO ((Table 1 y Figure 2).. By measuring the effects of reference compounds on larvae activity, we were able to estimate the concentration that could cause a 50% decrease in activity events values (AEC50), showing a strong linear correlation (R2 Q1 = 0.91) with the LC50 values obtained with the standard DarT test (Fig. 3) In this study, we demonstrate that global swimming behavior could be a simple readout for toxicity, easy to scale-up in automated experiments. This approach is potentially applicable for fast ecotoxicity assays and whole-organism high-throughput compound screening, reducing the time and money required to evaluate unknown samples and to identify leading pharmaceutical compounds.
…………………………………………………………………………………………………………………………..
J Appl Toxicol. 2014 Feb;34(2):214-9. doi: 10.1002/jat.2856. Epub 2013 Feb 11.
Darío Bicharaa,b, Nora B. Calcaterrab, Silvia Arranzb, Pablo Armasb and Sergio H. Simonettaa
