
Microbial communities that are exposed to sunlight typically share a series of adaptations to deal with the radiation they are exposed to, including efficient DNA repair systems, pigment production and protection against oxidative stress, which makes these environments good candidates for the search of new antioxidant microorganisms. In this research project, we isolated potential antioxidant pigmented bacteria from a dry and highly-irradiated extreme environment: solar panels. High throughput in vivo assays using Caenorhabditis elegans as an experimental model demonstrated the high antioxidant and ultraviolet-protection properties of these bacterial isolates that proved to be rich in carotenoids. Our results suggest that solar panels harbor a microbial community that includes strains with potential applications as antioxidants.
Methods
Worms were synchronized by isolating eggs from gravid adults at 20ºC. Synchronization was performed on NGM plates with E. coliOP50 as a negative control, E. coli OP50 plus vitamin C (vit C)at 20 mg/mL as a positive control, or E. coli OP50 plus the pigmented isolates (Table.1) in order to test antioxidant properties of the bacteria. The isolates were grown overnight in liquid LB medium at 28ºC and 180 rpm, optical density at 600 nm (OD600) was adjusted to 30 and to 60, and 50 uL of the bacterial suspension was added to the plates. The synchronized worms were incubated for a total of 3 days on the previously described plates, until reaching young adult stage. Young adult worms were collected and washed three times with M9 buffer, and finally resuspended in 100–200 uL of the buffer. Worms were then transferred by pipetting to 96-well plates (10–30 worms per well) containing M9 buffer. After transferring all the worms, hydrogen peroxide was added to the wells, reaching a final concentration of 1.2 mM of hydrogen peroxide. Mobility of the worms was measured with the WMicrotracker device during 60 min (four measurements of 15 min). In this experiment, data was collected in the form of “worm activity” (or relative locomotive activity), and was normalized by the number of worms in each well. All assays were performed with two biological replicates.
Results
In general, there was no significant differences in antioxidant activity between the worms incubated with the isolates at an OD of 30 or of 60, although a lower OD was beneficial for worm movement and, therefore, was the OD of choice for further experiments. After 30 min of incubation, PS30 did not display significant differences in activity per worm in comparison to the negative control, and PS10 displayed lower mobility than the negative control, indicating more worm mortality. On the other hand, incubation of the worms with PS1, PS13, PS21, PS75, PS17, PS47, PS19, PS20, PS63, and PS83 resulted in a higher protection of these worms against oxidative stress, with significant differences with respect to the negative control, and in some cases, with significantly higher protection in comparison to the positive control. In order to compare all experiments, an antioxidant index (AI) was calculated for each isolate by dividing the average activity per worm at 30 min when incubated with the isolate at OD 30 or 60 (the highest activity was used) by the average activity per worm of the positive control (Figure 1B). Nine out of the ten tested isolates displayed higher antioxidant activity than the positive control (AI > 1), although three of these (PS47, PS19, and PS20) could not be compared to the rest due to the worms being smaller and, in some cases, not correctly synchronized.
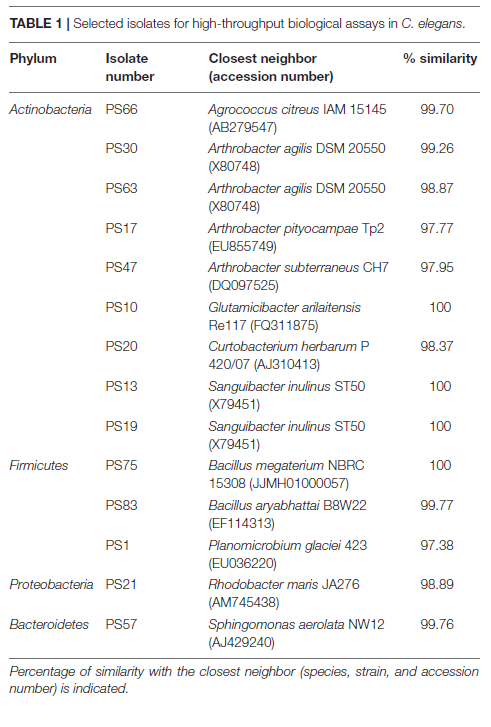
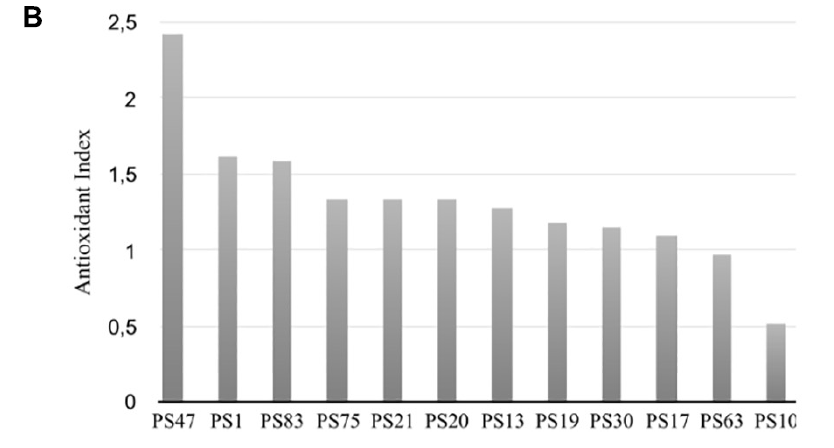
(at an OD600 of 30 or 60) of each isolate by the average activity of the positive control (vitC) after 30 min of incubation with hydrogen peroxide.
Front Microbiol. 2019 May 7;10:986. doi: 10.3389/fmicb.2019.00986. eCollection 2019.
Kristie Tanner, Patricia Martorell, Salvador Genovés, Daniel Ramón, Lorenzo Zacarías, María Jesús Rodrigo, Juli Peretó and Manuel Porcar.
