
In most organisms, lipid abundance is strictly regulated and sensitive to numerous physiological and environmental stimuli. One of the major effectors of lipid homeostasis is cyclic AMP dependent protein kinase (also known as protein kinase A [PKA]). PKA has been implicated in the response to starvation in C. elegans, where deprivation of nutrients results in elevated cAMP levels and consequent PKA activation. When activated, PKA then phosphorylates ATGL-1, a homolog of mammalian ATGL, enhancing its lipolytic activity on the surface of lipid drop lets. This enzyme is the rate-limiting step in triglyceride hydrolysis, and its activation results in generation of free fatty acids (FFAs) to maintain energy resources during periods of starvation. In C. elegans, the catalytic and regulatory subunits of PKA are encoded by kin-1 and kin-2, respectively. Both genes are highly expressed in body wall muscle cells, suggesting an important role in regulating muscle metabolism. In C. elegans, KIN-1, the ortholog of the catalytic PKA subunit, directly phosphorylates ATGL-1 to enhance lipolysis, consequently reducing fat stores. We therefore used an RNAi-based strategy (kin-2 RNAi) to constitutively activate PKA/KIN-1 and its downstream targets, including ATGL-1. In addition, we restricted the RNAi response to muscles by using a C. elegans strain that is sensitive to RNAi exclusively in body wall muscle cells (muscle-restricted RNAi [mrRNAi]), allowing us to address the global effects of enhanced PKA signaling uniquely in a single cell type.
Methods
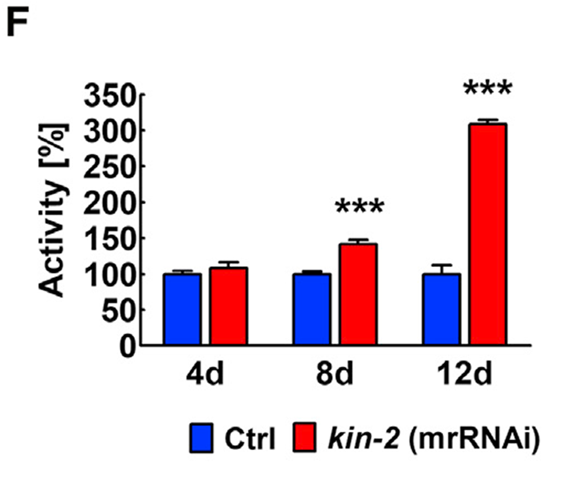
Quantification of Activity. Activity was quantified using a WMicroTracker machine (Wmicrotracker ONE, Phylum Tech) based on movement-mediated interruption of an infrared microbeam. Following RNAi treatment for day 4, 8, 12 after hatching, 10 animals per condition were transferred to a single well of a 96-well plate containing 200ul M9 buffer + 0.01% Tween 20 and movements were scored over the course of 10 hours. Experiments were done in triplicate. Data are represented as mean ± SD. Statistical analysis was performed using a two-way ANOVA and a Bonferroni post-test (*p < 0.05; **p < 0.01; ***p < 0.001).
Results
In contrast to the effects of global kin-2 suppression, we found that mrkin-2(RNAi) animals have improved locomotor activity at both young and older stages and are surprisingly longlived (Figures 1F). The activation of PKA exclusively in muscle cells confers lifespan-extending effects. This lifespan extension is likely a property that is unique to muscle because we did not observe this in any other cell type we investigated. Furthermore, our data are consistent with PKA acting non-cell-autonomously in the regulation of organismal aging through activation of its diverse downstream targets.
Cell Rep. 2019 Dec 24;29(13):4540-4552.e8. doi: 10.1016/j.celrep.2019.11.090.
Sebastian Schmeisser, Shaolin Li , Bertrand Bouchard, Matthieu Ruiz, Christine Des Rosiers, Richard Roy.
