
MAPK8IP3-related neurodevelopmental disorders are a spectrum of rare conditions caused by de novo mutations in the MAPK8IP3 gene that encodes the JIP3 protein. JIP3 is required for axonal transport of proteins and organelles between the soma and the synaptic terminal of neurons, a process critical for normal brain development and function.
Here we conduct thorough morphological, behavioral, and motility phenotyping in the JIP3 knockout zebrafish and identify locomotor deficits and morphological abnormalities. To identify treatment options, we used insights from expert clinicians and the artificial intelligence tool, mediKanren, to identify drug candidates hypothesized to improve patient symptoms or compensate for the loss of JIP3 at the molecular level. We then prioritized drugs that are FDA-approved, safe for children, and readily available. These collective efforts identified amantadine and levodopa as candidate therapies and rescued motor phenotypes associated with JIP3 loss-offunction in zebrafish.
Methods
Behavioral Phenotyping
JIP3 heterozygotes were set up in breeding tanks in a 1:1 ratio the night before spawning in a single tank with a divider, to ensure breeding occurred in the morning. Heterozygous in crosses allowed for an unbiased screening of all three genotypes: wild type (JIP3 +/+), heterozygous (JIP3 -/+) and homozygous JIP3 knockouts (JIP3 -/-) clutch mates. Fertilized zygotes were collected within 2 hours of spawning time and transferred to Petri dishes (100 embryos per dish) with approximately 200 milliliters of E3 solution. Larval fish were incubated AT 28°C and E3 was replenished daily until 6 days post fertilization (dpf). At 6 dpf experimental assays were performed.
Locomotor phenotypes were examined with the PhylumTech WMicrotracker ONE system and acquisition software, which allows for the assessment of 96 larvae at a time. Activity event parameters were defined as greater than 3% in the received light beam signal. Larvae were individually transferred to a 96-well flat-bottom plate using transfer pipettes. Wells were filled with E3 solution until they contained similar volumes. The larvae were allowed to acclimate in the room with the WMicroTracker ONE for 10 minutes prior to the assessment beginning. Events were measured in 30-minute intervals, over 2 hours, and averaged to calculate the average total locomotor activity. All assessments were completed during zebrafish light hours.
Drug treatment
All drug compounds were ordered from Sigma-Aldrich or as libraries from MedChem Express. Drugs not soluble in E3 water were dissolved in a small amount of 0.05% DMSO and then brought up to stock concentration in E3 water solution. Additional lower concentrations tested were diluted from stock. Concentrations were selected from prior toxicology reports in zebrafish or tested through trial and error; most candidate drugs were screened at 0.1-100 µM. Drugs with less bioavailability or solubility were tested at higher concentrations. Zebrafish were incubated in petri dishes filled with drug E3 water immediately after fertilization and drug water changes were completed daily to replenish drug concentrations. Microtracker assay was performed at 6 dpf. Abnormal phenotypes or lethality were recorded. Analyses were conducted using GraphPad Prism software LLC (Boston, MA). Behavioral comparisons across genotypes were completed using 1-way ANOVAs with Tukey’s test for multiple comparisons. Swim tunnel groups (wild-type male, wild-type female, JIP3 -/- male, JIP3 -/- female) were compared using multiple unpaired t-tests, and significance was defined as a p-value less than 0.05.
Results
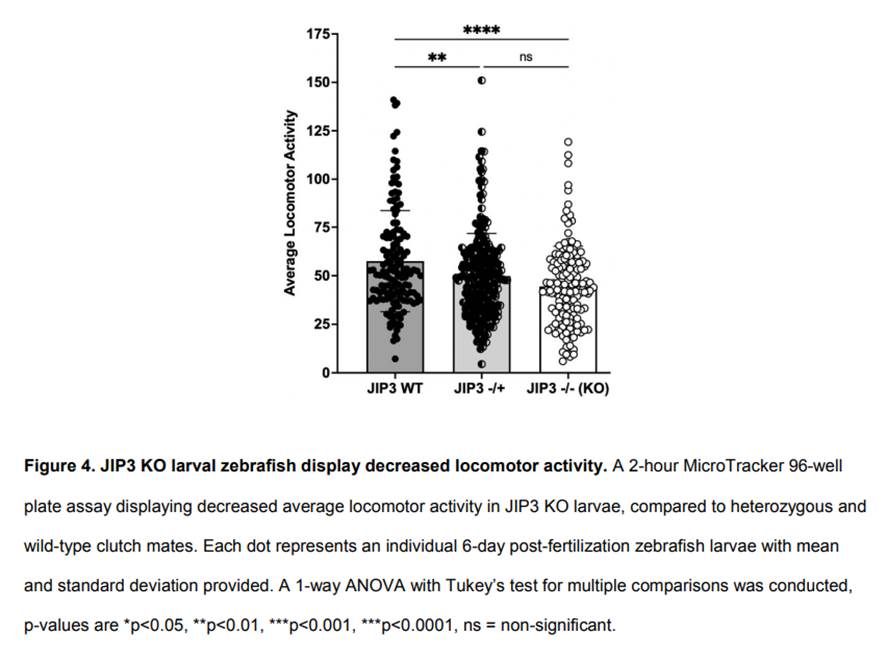
To assess how JIP3 loss-of-function alters locomotor behaviors, we assayed 6-day post-fertilization larvae Zebrafish. MicroTracker assays showed decreased average locomotor activity, compared to wild-type (p < 0.0001), and no difference was detected between JIP3 KO and heterozygous clutch mates (Figure 4). These data indicate that loss of JIP3 results in significant locomotor deficits in larval zebrafish.
We next began screening FDA-approved small molecules hypothesized to alleviate patient symptoms or target the disorder at the molecular level. These experiments showed that administration of amantadine or levodopa, immediately after fertilization, rescued average locomotor activity in JIP3 knockout larvae zebrafish (Figure 5). Both drugs lead to a slight increase in average locomotor activity in wild-type fish, but not to a significantly significant extent.
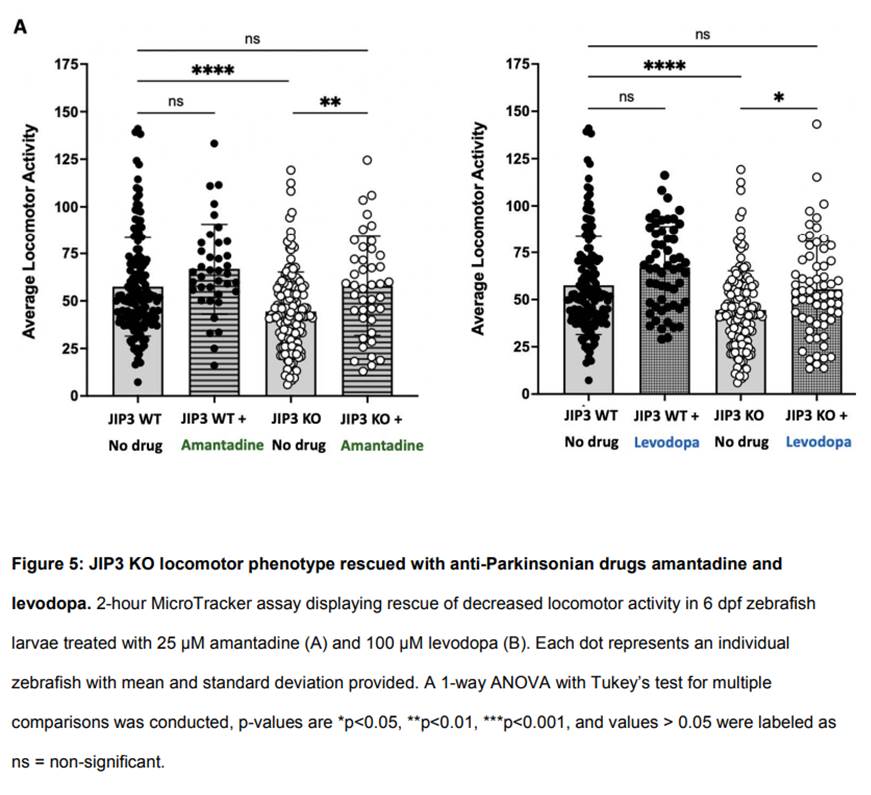
Here we found that levodopa and amantadine both rescued motor phenotypes in JIP3 KO zebrafish larvae, highlighting their potential for addressing motor dysfunction associated with the broader spectrum of MAPK8IP3-related disorders. One mechanistic hypothesis is that motor impairments from these disorders may be mitigated by enhancing dopaminergic neurotransmission during early development.
bioRxiv 2025.02.02.636112; doi: https://doi.org/10.1101/2025.02.02.636112
Aleksandra Foksinska, Paige Souder, Gabrielle Smith, Kinnsley Travis, Sienna Rucka, Addie Allred, Rebeca Bender, Jackie Brunson, Avary Lanier, Amber Glaze, Andrew Crouse, Matt Might, Camerron M. Crowder.
