The tiny C. elegans worm, just 1 mm in size and originally found in rotting fruit on the ground, was established as an animal model for developmental biology in the early 1970s by Sidney Brenner. From that starting point, the community has grown to thousands of researchers (with over 40,000 publications by 2024), and investigations on this simple organism have demonstrated how research on small organisms can contribute to our understanding of human health.
Brenner was awarded the Nobel Prize in 2002 “for his discoveries concerning genetic regulation of organ development and programmed cell death.” C. elegans was transparent, had all types of specialized cells, was easily manipulable, and reproduced quickly—a geneticist’s dream. For this reason, it’s not surprising that two postdoctoral students on different continents became fascinated with it. In the early 1980s, Victor Ambros and Gary Ruvkun met in the laboratory of Robert Horvitz (another of the 2002 Nobel laureates), working on a long-standing problem: why some ‘mutant’ worms developed abnormal structures and forms.
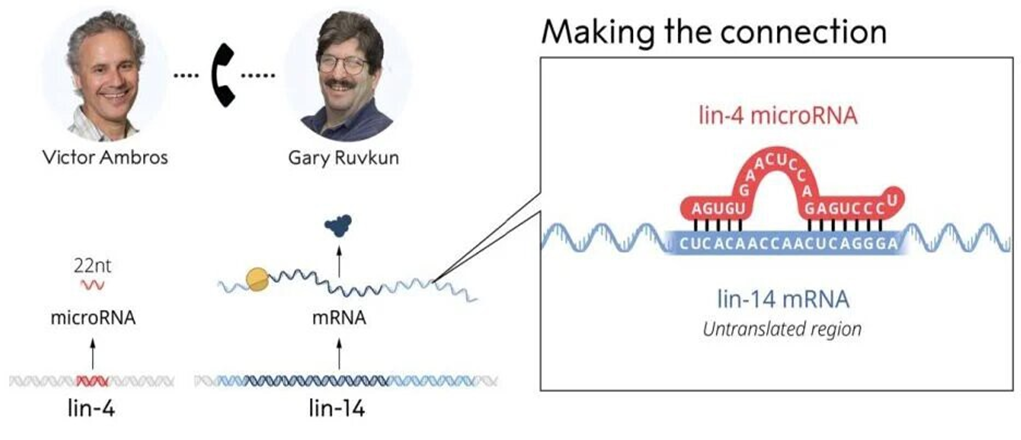
Their work initially focused on cloning and manipulating several types of mutant nematodes, but the solution remained elusive, and they completed their fellowships without solving it. They went their separate ways, with Ambros continuing at Harvard University and Ruvkun at Massachusetts General Hospital. Though close, each was working independently on a problem thought to be unsolvable. Ambros discovered very short pieces of RNA, though he didn’t know their function. Meanwhile, Ruvkun identified when certain genes were activated but couldn’t understand what activated or blocked them.
On June 11, 1992, nearly a decade after they started collaborating, they met again and shared their discoveries. The essential question they were trying to answer was: What ensures that only the correct set of genes is active in each cell type?
Ambros had one part of the puzzle, and Ruvkun had the other. Together, they uncovered an entirely new level of genetic regulation that would prove crucial years later. Initially, few paid attention, thinking the mechanism might be unique to C. elegans. It took nearly another decade for Ruvkun’s lab to identify another instance of microRNA, this time in a gene common to all living organisms. This breakthrough sparked a wave of research: dozens of labs began studying microRNAs, revealing that abnormal microRNA regulation could contribute to cancer and that mutations in microRNA-coding genes are linked to conditions such as congenital hearing loss, eye disorders, and skeletal abnormalities.
Today, many significant therapeutic advances rely on their work. And now, they have a Nobel Prize to honor this groundbreaking discovery.
List of Previous Nobel lectures:
The Nobel Prize in Physiology or Medicine 2002
The Nobel Prize in Physiology or Medicine 2006
The Nobel Prize in Chemistry 2008
