
Plant-parasitic nematodes (PPN) are economically important pests responsible for substantial losses in agriculture. Researchers focusing on plant-parasitic nematodes often need to assess basic parameters such as their motility, viability, and reproduction. Traditionally, these assays involve visually counting juveniles and eggs under a dissecting microscope, making this investigation time-consuming and laborious. In this study, we established a procedure to efficiently determine the motility of two plant-parasitic nematode species, Heterodera schachtii and Ditylenchus destructor, using the WMicrotracker ONE platform. Additionally, we demonstrated that hatching of the cyst nematode H. schachtii can be evaluated using both the WMicrotracker ONE and by assessing the enzymatic activity of chitinase produced during hatching. Several high-throughput methods for performing similar assays have been optimized for the model nematode Caenorhabditis elegans and, to some extent, for some species of mammalian parasitic nematodes. Surprisingly, attempts to adapt such methods for PPN appear to be relatively rare. WMicrotracker ONE provided reliable results, suggesting that the system is compatible with various PPN species.
Methods
Collecting nematodes for experiments
The motile H. schachtii J2 required for the tests were collected from funnels filled with 3 mM ZnCl2 to increase the hatching rate of the nematodes. Approximately 300 cysts were placed in a sieve (60 μm mesh size) in the funnel so that it was approximately half covered with the liquid. The hatched J2 passed through the sieve and settled at the exit of the funnel, which led to a silicone tube closed with a clip. By opening the clip, the hatched J2 could be easily collected. The best time to collect a healthy population of J2 for the experiment was between 3 and 10 days after dissection of the cysts. Juveniles can be collected repeatedly from a funnel.
For experiments with D. destructor, 5 ml of sterile ddH2O was added to plates containing infected carrot discs. Nematodes naturally migrate from the discs into the water. To increase the yield, the discs were partially submerged in water for approximately 30 min. The liquid containing nematodes was transferred from the plates to Eppendorf tubes. In case any debris from the carrot discs was collected, the nematodes were washed several times with water prior to the experiment. Populations containing a mixture of different developmental stages were used for the experiments.
The concentration of nematodes was determined by counting the number of living nematodes in 3 10 μL drops. The suspension was further diluted with sterile ddH2O to achieve the desired final concentration.
Evaluation of nematode motility using WMicroTracker ONE
The suspension was distributed into U-bottom 96-well plates (54 μL per well). The plates were kept in an incubator set to 20°C for 20–30 min prior to the measurement to allow the nematodes to settle on the bottom of the wells. Afterwards, the plates were placed into WMicrotracker ONE device, and the initial motility of the worms was recorded for 30 minutes. Six microliters of the tested control chemicals (sodium hypochlorite and sodium azide at a concentration 10 times greater than the desired final concentration) or sterile ddH2O water was added to each well (at least 4 wells per condition), and the motility of the populations was remeasured using WMicrotracker ONE at different time points. Between the measurements, the experimental plates were sealed with parafilm or PCR seal, kept at 20°C, and gently shaken on an orbital shaker (150 rpm) to ensure aeration of the suspension.
Evaluation of Heterodera schachtii hatching
Measuring the movement of J2 emerging from cysts using WMicrotracker ONE: The wells of a U-bottom 96-well plate were filled with 54 μL of sterile ddH2O or 3 mM ZnCl2. Three cysts were collected from the maintenance plate and placed into each well, while trying to ensure that cysts of similar size and colour were evenly distributed across the wells. After measuring the initial motility on WMicrotracker ONE (which should be close to 0 because no juveniles have yet emerged), 6 μL of the control chemical (ethanol at a concentration 10 times higher than the desired final concentration) or sterile ddH2O was added to each well. Due to the greater inherent variability of this assay, at least 8 wells per condition were used. The experimental plates were kept under the same conditions and remeasured a tdifferent time points.
Measuring hatching using WMicrotracker ONE: Approximately 300 cysts were collected from maintenance plates or retrieved from funnels previously used for other experiments and placed into a 100 ml glass bottle filled with 3–5 ml of sterile ddH2O or 3mM ZnCl2. A medium-sized stirring bar was added to the bottle, and the cysts were crushed on a magnetic stirrer (1000 rpm, 5 min). The suspension was passed through a sieve (30 μm pore size) to remove smaller debris and some J2 that had already hatched inside of the cysts. The sieve was placed bottom up on a piece of mesh (116 μm pore size) and washed with 3–5 ml of ddH2O. The liquid passing through the mesh was collected. This step removes larger debris. The final suspension was enriched in eggs but was not completely clean, as some J2 and mid-sized debris were also collected. The concentration of eggs was determined by counting the number of intact eggs in three 10 μL drops under a microscope. Approximately 50 eggs per well were used. The experimental plates were prepared, stored, and measured.
Results
WMicrotracker ONE is a suitable tool for measuring the movement of plant-parasitic nematodes.
Our experiments (Fig. 1) show that the platform can be used for PPN, including both migratory species and motile developmental stages of sedentary species, which are generally considered the most economically important. We measured the motility of the treated and control nematodes for 2 hours immediately after the addition of the compounds and again 3 days after treatment (Fig. 1). As expected, both species showed a rapid decline in movement after exposure to both substances. After 30 minutes, the activity was reduced by73.8% (from mean 258.9 activity counts to 67.7) in populations of D. destructor treated with NaN3, 84.9%(from mean 243.9 activity counts to 36.9) in D. destructor treated with NaClO, 98.9% (from mean 227.8activity counts to only 2.4) in H. schachtii exposed to NaN3, and 79.7% (from mean 241.6 activity counts to 49) in H. schachtii in NaClO. Less than 1 mean activity count could be detected in populations of both nematode species treated with both substances after 3 days. At the same time, nematode motility in the controls remained consistent, with no decrease in activity counts detected in the short-term experiment and only a small decrease in motility (6–8% on average) after 3 days. According to our results, approximately 100–150 worms per well should be used for smaller and less active nematodes such as H. schachtii J2. For D. destructor and other more active PPN species, 30–50 nematodes per well are sufficient. Using plates with round bottoms allows nematodes to accumulate more closely and further stimulate each other’s movement by touch, resulting in higher detected activity counts than in plates with flat bottoms. The assay is very efficient and easy to evaluate, especially in comparison to the visual counting of moving nematodes under a microscope.
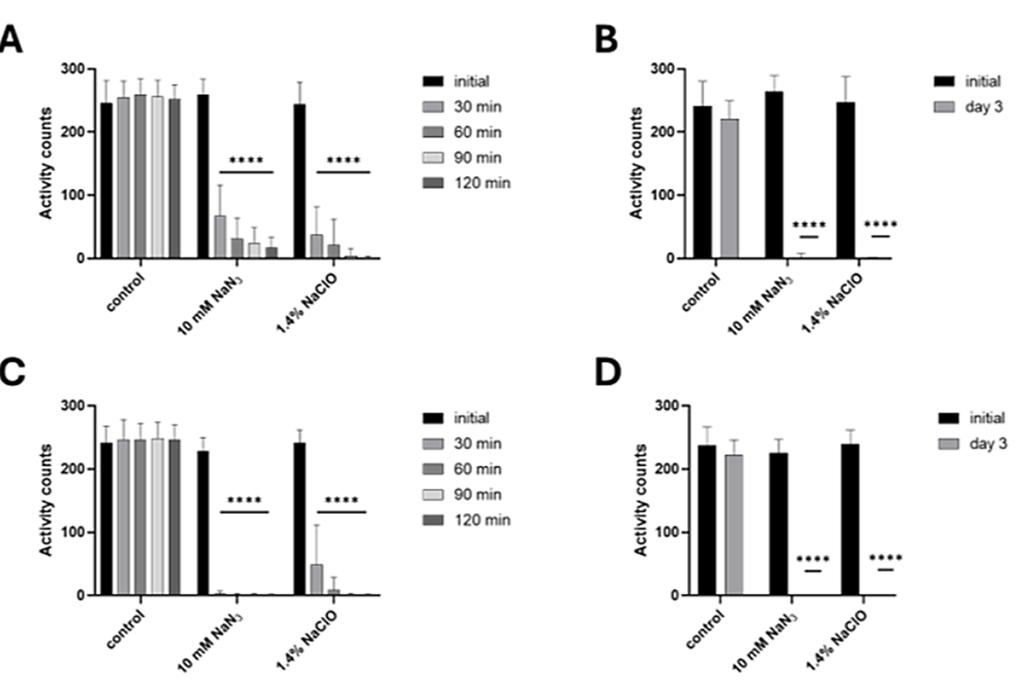
Measuring the motility of plant-parasitic nematodes using the WMicrotracker ONE. A) Effect of short (2- hour) exposure to 2 toxic chemicals (NaN3 and NaClO) on the movement of Ditylenchus destructor (mixed age). The graph shows the means + SDs from 6 biological (at least 29 technical) replicates. B) The effect of longer (3 days) exposure to 2 toxic chemicals on the movement of D. destructor (mixed age). The graph shows the means + SDs from 8 biological (at least 55 technical) replicates. C) The effect of short (2-hour) exposure to 2 toxic chemicals on the movement of Heterodera schachtii J2. The graph shows the means + SDs from 7 biological (at least 44 technical) replicates. D) The effect of longer (3 days) exposure to 2 toxic chemicals on the movement of H. schachtii J2. The graph shows the means + SDs from 4 biological (at least 29 technical) replicates. In all graphs, asterisks indicate statistically significant differences compared to the negative control at the indicated time points (**** p < 0.0001; twoway RM ANOVA, Dunnett´s multiple comparison test).
Measuring the motility and hatching of Heterodera schachtii using the WMicrotracker ONE.
We tested two different setups—measuring the increase in motility in wells with intact cysts and in wells containing the egg mixtures prepared in the same way as for the chitinase assay. In both cases, we again compared the data for populations incubated in water and in ZnCl2. Here, the reported stimulatory effect of zinc salt on the hatching of Heterodera spp. was clearly apparent (Fig. 3). In wells containing eggs incubated in ZnCl2, we detected a 60% increase in motility compared to water (195.9 mean activity counts compared to 78.8). For intact cysts, the difference was 44% (229.9 mean activity counts compared to 128.4). Therefore, we used ZnCl2 in all subsequent experiments.
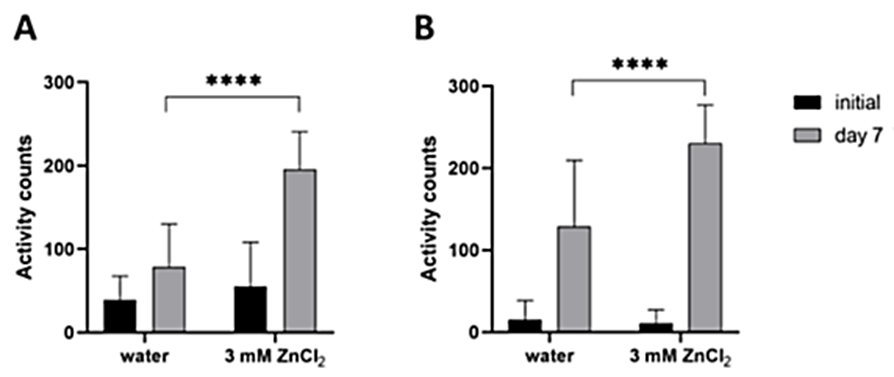
Heterodera schachtii eggs and cysts incubated in ZnCl2 for 7 days showed significantly greater activity than those incubated in water (measured by WMicrotracker ONE). A) Results from isolated eggs, 50 per well. The data are shown as the means + SDs from 5 biological (at least 16 technical) replicates. B)Results from intact cysts, 3 per well. The data are shown as the means + SDs from 3 biological (28 technical) replicates. In both graphs, asterisks indicate statistically significant differences compared to the water control group (**** p < 0.0001; two-way RM ANOVA, Dunnett multiple comparison test).
To validate the robustness of the two assay setups, various concentrations of ethanol were used as a positive control, demonstrating the dose-dependent effect of the substance at various time points (Fig. 4).
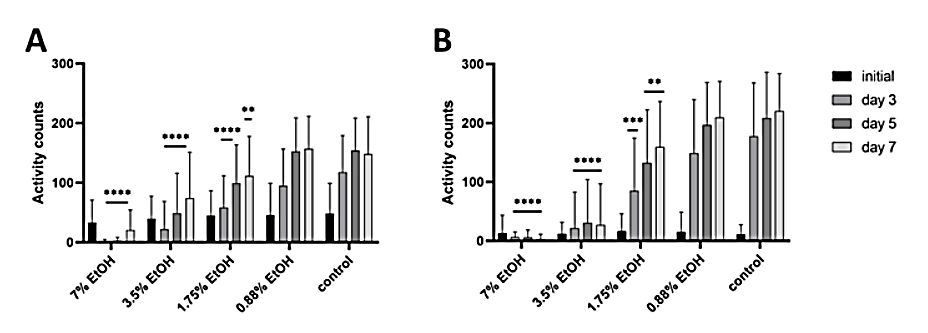
Effect of different concentrations of ethanol (7% – 0.88%) on Heterodera schachtii activity measured with WMicrotracker ONE. A) Results from isolated eggs, 50 per well, incubated in ZnCl2. The data are shown as the means + SDs from 8 biological (64 technical) replicates. B) Results from intact cysts, 3 per well, incubated in ZnCl2. The data are shown as the means + SDs from 4 biological (32 technical) replicates. In both graphs, asterisks indicate statistically significant differences compared to the water-treated control at the indicated time points (**** p < 0.0001; *** 0.001 > p > 0.0001; ** 0.05 > p > 0.001; two-way RM ANOVA, Dunnett´s multiple comparison test).
For both setups, we observed greater variability among the technical replicates, while the effect remained consistent among the biological replicates. We assume that this is due to inherent variability among the cysts and eggs and recommend using at least 8 technical replicates per condition to mitigate this issue. Furthermore, we noticed that the age of the plates from which the cysts were collected can significantly influence how soon hatching starts. Therefore, we recommend using maintenance plates of approximately the same age across all biological replicates.
In the setup with intact cysts, we recommend using 3 cysts per well. Using fewer than 3 further increases the variability among technical replicates, while more cysts in the well seem to interfere with the instrument´s ability to measure the activity properly. For the egg suspension, we recommend using 50 eggs per well. The suspension obtained from crushed cysts, while enriched in eggs, also contained some J2 and mid-size debris. The presence of J2 at the beginning of the test results in some activity being detected in the wells during the initial measurement prior to adding the substances.
The difference between the positive and negative controls was reproducible and statistically significant in all biological replicates. For intact cysts, we observed a gradual increase in activity counts from approximately 10–16 during the initial measurement in all conditions to 220.1 mean activity counts in control wells after 1 week. For ethanol-treated cysts, the values obtained at the same time point were 209.2, 159.2, 26.7 and 2.9 for 0.88, 1.75, 3.5 and 7% ethanol, respectively. The effect was dose-dependent and ranged from none or marginal to severe. For isolated eggs, the initial mean activity counts ranged from approximately 33 to 50 under all conditions for the reasons outlined above. The movement rates in
the wells gradually increased to approximately 150 mean activity counts at days 5 and 7 in both the control wells and the wells treated with 0.88% ethanol. A less prominent increase, to 111.6 and 73.8 mean activity counts after 1 week, was apparent in populations treated with 1.75 and 3.5% ethanol, respectively. The addition of 7% ethanol led to a decrease in the mean activity counts after 1 week compared to the initial value, to only 20.1.
From our experiments, we conclude that the developed methods can also be used with other economically important PPN, either in the presented form or with minor species-specific modifications. The methods are based on the detection of nematode movement in multiwell plates and the quantification of enzyme activity using a fluorogenic substrate. Inspired by protocols previously optimized for the model C. elegans and other nematodes, they also proved to be compatible with PPN species. These methods offer a more efficient alternative to commonly used approaches. They can find use in various areas of basic and applied research as well as in laboratories focused on PPN monitoring.
Research Square. April 2024. https://doi.org/10.21203/rs.3.rs-4235543/v1
Alena Kadlecová, Romana Hendrychová, Tomáš Jirsa, Václav Čermák, Mengmeng Huang, Florian M.W. Grundler, A. Sylvia S. Schleker.
