
For the past 40 years or so, oxidative stress has been increasingly recognized as a contributing factor in aging and in various forms of pathophysiology generally associated with aging. Research in C.elegans has contributed to understand many factors involved in aging, as well as the discovery of new molecules that help to reduce free radicals and oxidative stress damage.
In this post we intend to give an example of protocol for conducting a long term experiment of oxidative stress in C.elegans. Here are the steps to do it:
Protocol Brief:
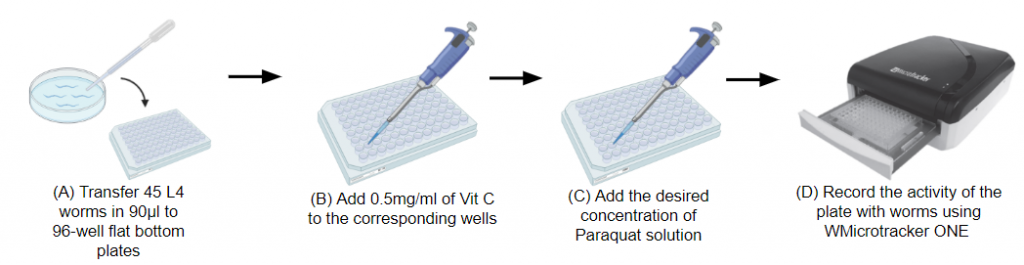
Steps to collect worms:
- Gradually grow C.elegans on NGM plates from synchronization with Sodium Hypochlorite. 2 plates containing 5000 worms w / will suffice.
2. Wash the early stage L4 synchronized worms with an M9 buffer or water from an agar plate.
3. Precipitate the worms by centrifugation discard the supernatant and add 5 ml of liquid culture medium.
Author’s note about the Liquid Culture Medium:
Depending on availability and preference, any of the following media can be used:
a) 3PY medium (3% soy peptone, 3% yeast extract [Generally called AXM medium: See Lenaerts et al] + 50uM-100uM FUdR + Antibiotics (streptomycin 200ug / ml + 20ug / ml Kanamycin)
b) S Basal media + UV Killed bacteria (killed by heat of paraformaldehyde can also works [See Beydoun et al])* + 50uM-100uM FUdR + Antibiotics (streptomycin 200ug / ml + 20ug / ml Kanamycin).
*Use always the same concentration of bacteria in your set of experiments (As example final OD600 = 0.4).
c) CeMM or CeHR axenic medium + 50uM-100uM FUdR + Antibiotics (streptomycin 200ug / ml + 20ug / ml Kanamycin)
The composition of the liquid medium is crucial, as the components may chelate and impact the oxidative/antioxidant properties of compounds, potentially altering their effective concentration.
The use of fluorodeoxyuridine (FUdR) inhibits the embryonic development of worms, preventing egg hatching. Sterile nematode strains can be employed for large screenings. Note that some manuscript reviewers may request the inclusion of N2 worms as a reference control at some point.
4. Count the number of worms / 5 ul and bring to volume to obtain a 12 ml suspension containing 35 to 50 worms / 90 ul of liquid culture medium.
Fractionation step and incubation with compounds.
5. Split 90 μl of the worm suspension into each well of the 96-well plate preferably using multichannel pipette.
6. Add 10 μl of compounds with potential antioxidant power to each well. Use technical quadruplicates if at all possible. Vitamin C (10 mg / ml) can be used as a positive control.
7. Seal the multi-well plate with parafilm® and place it at 20 ° C until the following day in case of pre-exposure overnight (recommended).
8. The next day (post-overnight) measure the plaque on the WMicrotracker and record the activity for at least 120 minutes (“Basal Activity”).
9. Submit the worms to oxidative stress shock: Place 11 μl of paraquat, 100 mM, 200 mM and 400 mM and 11 μl of K buffer solution in wells -VitC and + VitC (4 per condition). Re-seal the plate.
Insert the plate into the WMicrotracker and record the continuous activity for 6000 min.
10. At the end, generate the data report from blocks of 240 min. Relativize the activity reported against the Basal Activity (mathematically divide each of the measurements by the measurement of time 0)
11. Graph the relative activity vs time in excel.
If everything worked well, we should get a chart like the one shown below:
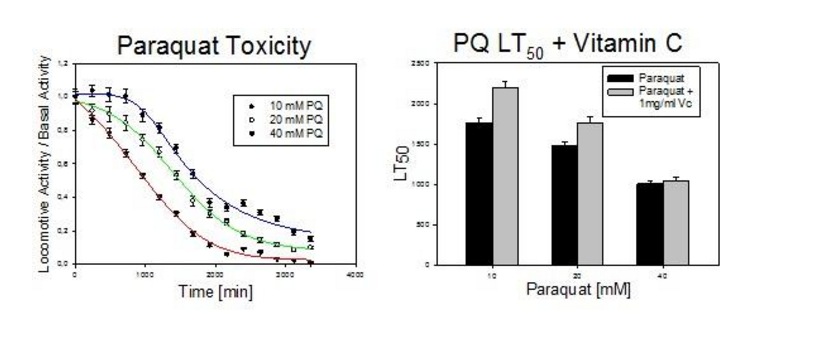
Finally, it is advisable to visually check the plates in order to discard wells that could be contaminated.
